
- EN
- DE
myoncare connects the global healthcare ecosystem - from hospitals to pharma, medtech, and payers - enabling continuous, data-driven care.


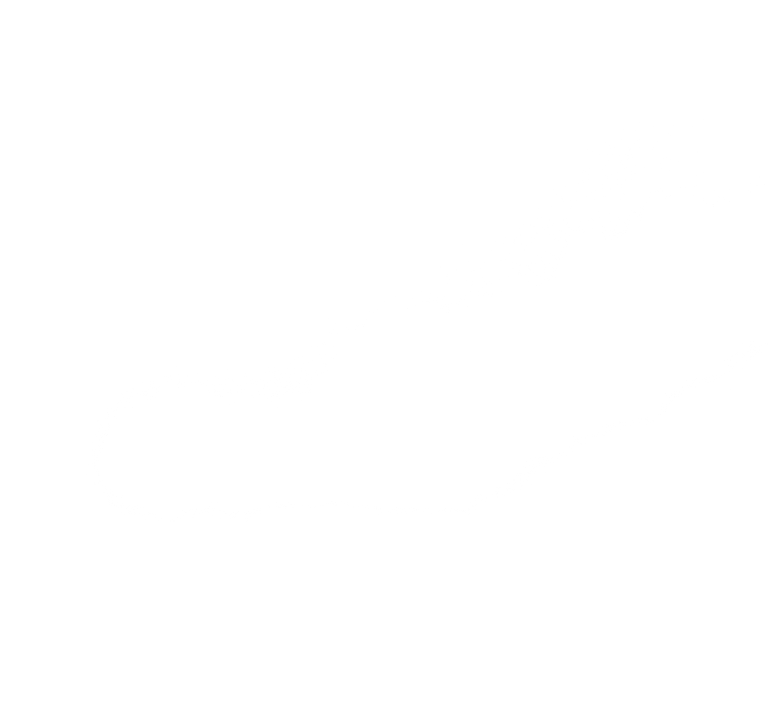
Virtually connect providers across all service levels.
myoncare is prepared to support the European Health Data Space through secure, interoperable, and patient-driven data exchange. Learn more
Integrating all stakeholders around the patient - so the right action happens at the right time
Benefit from a comprehensive compliance network to ensure adherence to regulators.

myoncare seamlessly connects prevention, chronic disease management, and peri-interventional care - helping care providers deliver better outcomes and reduce costs.
Empower patients to stay healthy - supported by insurers and employers who value proactive care.

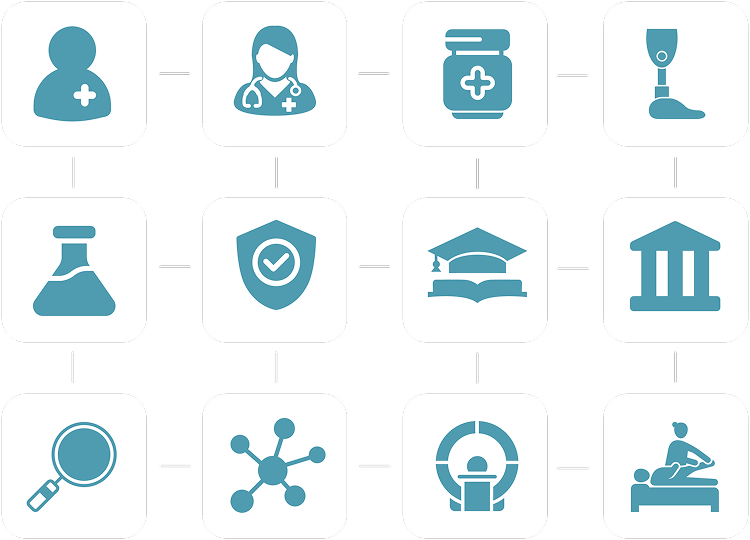
Reduce complications, shorten hospital stays, and enable seamless recovery with remote monitoring and communication.

From diabetes to hypertension - monitor, educate, and guide patients with digital pathways built on real-world data.
Trusted By






















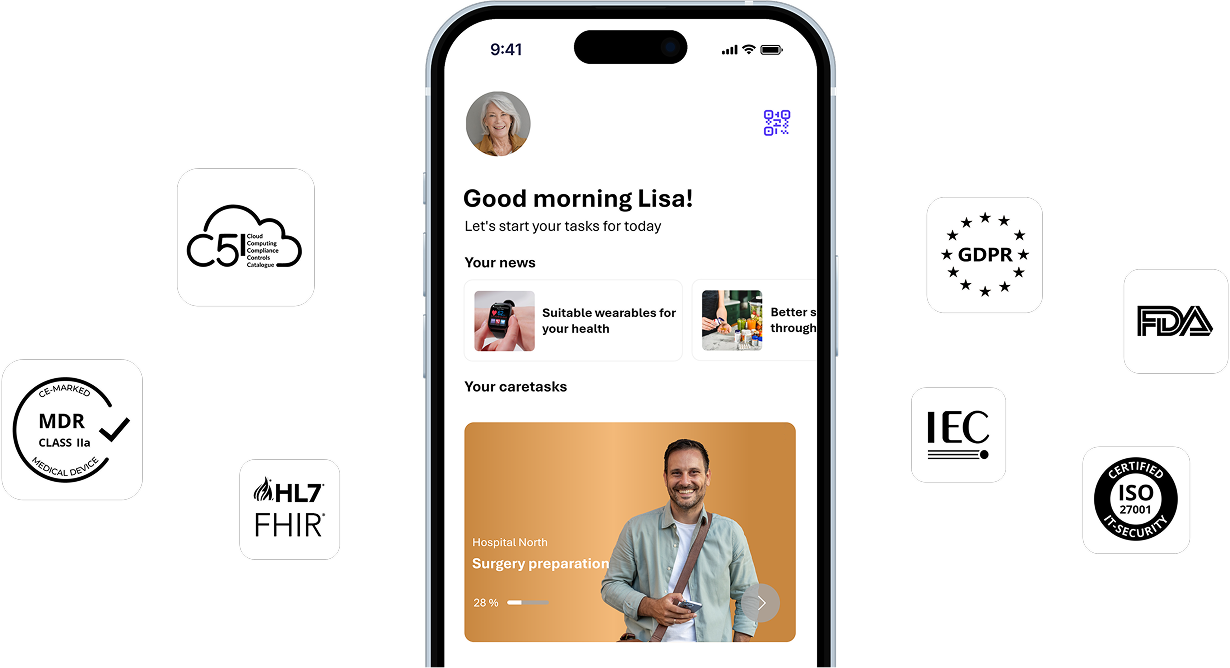
One platform connecting patients, care teams, and data. myoncare automates risk assessments, health monitoring, reminders, education, and telecommunication - all in one compliant infrastructure.
to get the tech, compliance, and infrastructure right
– so you don’t have to.

with our ai-powered content management system & pathway builder.
Choose from numerous modules to create customized solutions:

Designed to meet the highest standards for compliance, interoperability, flexibility, and research-driven collaboration.
The content management system is the heart of myoncare. Using the CMS, content can be easily created, stored with automation rules and combined into treatment paths. The user interface of the CMS has been designed to be particularly intuitive so that it can also be easily operated by users without programming knowledge.